Introduction
Werner Heisenberg a German physicist in 1927 stated uncertainty principle which is the consequence of dual behaviour of matter and radiation.
It states that it is impossible to determine simulataneously, the exact position and exact momentum( or velocity) of an electron.
Heisenberg's Uncertainty Principle states that there is inherent uncertainty in the act of measuring a variable of a particle. Commonly applied to the position and momentum of a particle, the principle states that the more precisely the position is known the more uncertain.
the momentum is and vice versa. This is contrary to classical Newtonian physics which holds all variables of particles to be measurable to an arbitrary uncertainty given good enough equipment. The Heisenberg Uncertainty Principle is a fundamental theory in quantum mechanics that defines why a scientist cannot measure multiple quantum variables simultaneously. Until the dawn of quantum mechanics, it was held as a fact that all variables of an object could be known to exact precision simultaneously for a given moment. Newtonian physics placed no limits on how better procedures and techniques could reduce measurement uncertainty so that it was conceivable that with proper care and accuracy all information could be defined. Heisenberg made the bold proposition that there is a lower limit to this precision making our knowledge of a particle inherently uncertain.the precise momentum of the particle, it is impossible to know the precise position, and vice versa.
The fundamental law comes into play in the quantum world because subatomic particles can behave like waves. A common misconception about the uncertainty principle in quantum physics is that it implies our measurements are uncertain or inaccurate. In fact, uncertainty is an inherent aspect of anything with wave-like behavior.
Mathematically ,it can be given as in equation.
significance of uncertainity principle
One of the important implications of the Heisenberg Uncertainty Principle is that it rules out existence of definite path or trajectories of electrons and other similar particles.
The tragic tree of an object is determine by its location and velocity at various moments.
If we know if where a body is at a particular instant and if we also known its velocity and the forces acting on it at that instant we can tell where the body would be some time later we there for conclude that the position of an object and its velocity fixed as an electron it is not possible simultaneously to determine the position and velocity at any given instant to an arbitrary degree of precision, it is not possible to talk of the trajectory of an electron.
The effect of Heisenberg Uncertainty Principle is significant only for motion of microscopic objecjs and is negligible for that macroscopic objects
Schrodinger equation :-
Quantum mechanics, as developed by Erwin Schrodinger in 1926, is based on the wave motion associated with the particles. For the wave motion of the electron in the three dimensional space around the nucleus, he put forward an equation known as Schrondinger wave equations.
When schrondinger equation is solved for hydrogen atom , the solution gives the possible energy levels the electron can occupy and the corresponding wave function (s) of the electron associated with each energy level.
Schrondinger wave equations:-
in short schrondinger wave equationis written as :-
HΨ=EΨ
Where H is a mathematical operator called Hamiltonian opretor.
The solution of Schrodinger wave equation for an electron in an atom gives the value of E and Ψ .The value of E represents the quantised value of energy which the electron in the atom can have. The corresponding value of ψ are called wave functions.
Aufbau Principle :-
The word 'Afbau' in German means building up'.
The building up of the orbitals means the filling up of orbitals with electrons.
The Aufbau principle, sometimes known as the Aufbau rule,
asserts that when an atom or ion is in its ground state, electrons occupy subshells with the lowest possible energy first, then subshells with higher energy. The 1s subshell for example, is occupied before the 2s subshell.
An atom’s or ion’s electrons form the most stable electron configuration feasible in this fashion.
The phosphorus atom, for example, has the configuration 1s2 2s2 2p6 3s2 3p3, indicating that the 1s subshell possesses two electrons, and so on.
Electrons enter the subshells of atoms in the increasing order of energy.
The following is the increasing order of energies of the subshells of atoms:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
The number before s, p, d, and f word is the main quantum number of electrons of its subshells.
It is also called the serial number of shells or shells of atoms. As the Aufbau principle says lower orbitals are filled first before higher orbitals.
The oder of the filling is presented in the digram
The exclusion principle given by the Austrian scientist Wolfgang Pauli in 1926.
No two electrons in an atom can have the same set of four quantam numbers.
Exclusion can also be stated as:-
Only two electrons may exist in the same orbital and these electrons must have opposite spin.
In chemistry, Pauli’s Exclusion Principle, along with Afbau Principal and Hund’s Rule, is one of the most significant principles. Pauli’s Exclusion Principle essentially helps us comprehend the electron configurations in atom and molecules and also provides an explanation for the periodic table's classification of elements.
Pauli's Exclusion Principle has two rules :-
1. Two electrons occupy the same orbital.
2. Two electrons in the same orbital have opposing spins or are antiparaller.
Hund's Rule :-
Hund’s Rule of maximum multiplicity provides a method for arranging electrons in orbitals of a subshell.
This rule deals with filling of electrons into the orbitals belonging to the same subshell ( that is ,orbitalsof equal energy,called degenerate orbitals).
The Aufbau rule works according to the energy level of the subshells and determines the lowest energy subshell to be filled first. Each subshell (s,p,d,f) has a different number of orbitals. Electrons filling in the orbitals of the same subshell follows the guidelines as stated by Hund’s rule. electrons with the same charge repel each other if they are in the same orbital. Repulsion can be reduced by moving two electrons as far apart as possible by occupying different degenerate orbitals or having parallel spins (in different orbitals).
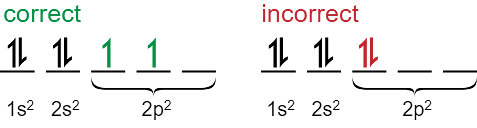
Hund’s Rule
The rule includes two basic parameters.
- Before completely filling a subshell, every orbital in that subshell must be singly filled.
- For maximum multiplicity, all electrons are assigned the same spin in orbitals with single occupancy.
- Each atom has subshells and a subshell can have a different number of orbitals. The Aufbau principle determines that the lowest energy orbital gets filled first. But an anomaly arises in the case of orbitals of the same subshell. The orbitals of the same subshell have the same energy. Hund’s rule comes into play here.
The following diagram illustrates how the 2p orbitals get filled up subsequently:
According to Hund’s rule of maximum multiplicity, all electrons while entering a subshell, fill up the orbitals of the subshell singly i.e. unpaired electrons occupy all the orbitals first. All such electrons are having the same spin so as to maximise the multiplicity of spin. According to the rule, the lowest energy term in the electronic configuration will be the one with the highest value of spin multiplicity.
Example of hund's rule :-
Let us examine how Hund's rule influences the electronic configuration of degenerate orbitals in the following atoms:
Nitrogen atom electronic configuration:- Oxygen atom electronic configuration:- Sodium atom electronic configuration:-









Comments
Post a Comment